High-throughput screening of large volumes of whole blood using structured illumination and fluorescent on-chip imaging published in Lab on a Chip (2012)
By S.A. Arpali , C. Arpali , A.F. Coskun ,
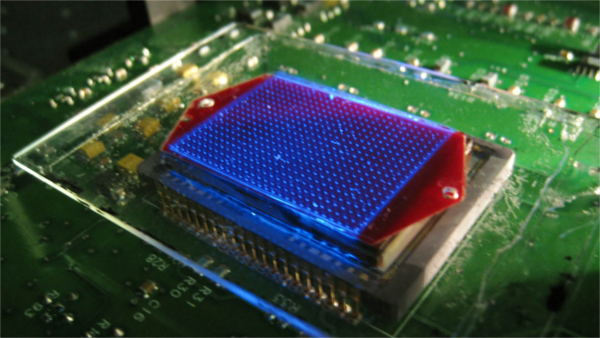
Undiluted blood samples are difficult to image in large volumes since blood constitutes a highly absorbing and scattering medium. As a result of this limitation, optical imaging of rare cells (e.g., circulating tumour cells) within unprocessed whole blood remains a challenge, demanding the use of special microfluidic technologies. Here we demonstrate a new fluorescent on-chip imaging modality that can rapidly screen large volumes ofabsorbing and scattering media, such as undiluted whole blood samples, for detection of fluorescent micro-objects at low concentrations (forexample ?50–100 particles/mL). In this high-throughput imaging modality, a large area microfluidic device (e.g., 7–18 cm2), whichcontains for example 0.3–0.7 mL of undiluted whole blood sample, is directly positioned onto a wide-field opto-electronic sensor-array such that the fluorescent emission within the microchannel can be detected without the use of any imaging lenses. This microfluidic device is then illuminated and laterally scanned with an array of Gaussian excitation spots, which is generated through a spatial light modulator. For each scanning position of this excitation array, a lensfree fluorescent image of the blood sample is captured using the opto-electronic sensor-array, resulting in a sequence of images (e.g., 144 lensfree frames captured in 36 s) for the same sample chip. Digitally merging these lensfree fluorescent images based on a maximumintensity projection (MIP) algorithm enabled us to significantly boost the signal-to-noise ratio (SNR) and contrast of the fluorescent micro-objectswithin whole blood, which normally remain undetected (i.e., hidden) using conventional uniform excitation schemes, involving plane waveillumination. This high-throughput on-chip imaging platform based on structured excitation could be useful for rare cell research by enabling rapid screening of large volume microfluidic devices that process whole blood and other optically dense media.